☰ ATSU Navigation

PCORI's new free, on-demand training, Research Fundamentals, allows users to learn about the health research process and become involved in patient-centered outcomes research.
The training package’s advantages include:
NIH advertises availability of grants through funding opportunity announcements (FOAs), of which a NOSI is one type. This type of FOA highlights a scientific area of interest for the participating institutes/centers (ICs). In June 2019, NIH formalized and expanded the use of NOSIs to replace topic-specific Program Announcements (PAs).
Some other things to know:
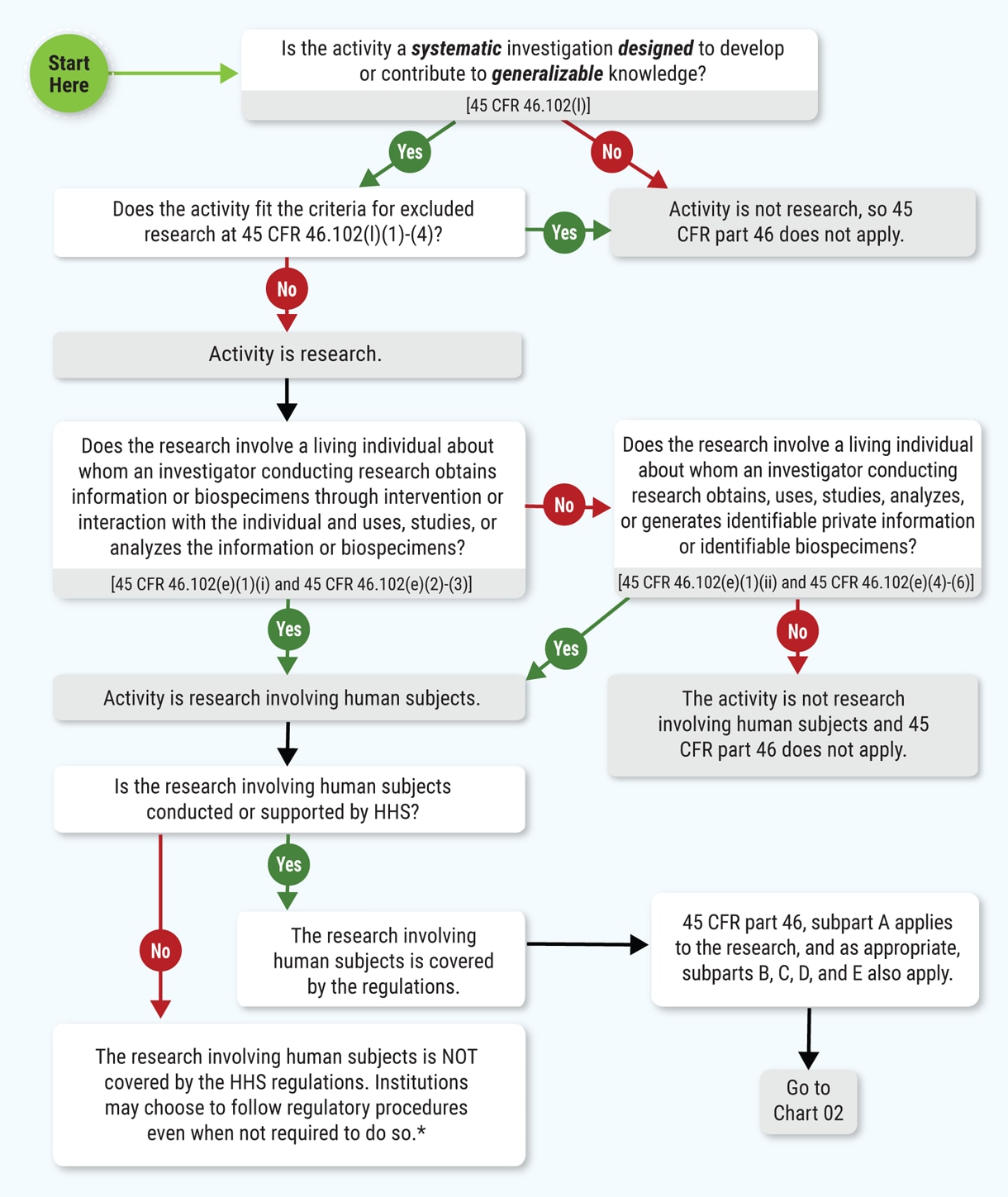
Having trouble deciding if your proposed activity qualifies as research involving human subjects? The Office for Human Research Protections has posted a set of decision charts to help you in deciding whether:
Decision charts are available for requirements consistent with pre-2018 and 2018 regulations.

Listen to NIH’s 13-minute All About Grants podcast to learn more about doing human subjects research (transcript available here).
Lyndi Lahl, RN, a Human Subjects Officer with the NIH Office of Extramural Research, will help you understand what exactly is meant by “human subjects research,” its relation to the recently revised Common Rule, what research may be exempted, what institutions need to have in place, where to find important resources, and more.
