☰ ATSU Navigation
General News
Formulating PCORI's Approach to New Priority Research Areas
As part of new legislation that reauthorized PCORI’s funding for 10 years, Congress included 2 new research priority areas to address: (1) strategies for improving maternal mortality and (2) improving health for individuals with intellectual and/or developmental disabilities. In a recent blog post, Executive director Nakela L. Cook, MD, MPH, discusses PCORI’s plans over the next decade.
Executive Director Spoke at Research!America Forum

On September 10, Dr. Cook held a Fireside Chat with Nancy Brown, CEO of the American Heart Association, at Research!America’s 2020 Virtual National Health Research Forum, Straight Talk: Securing a Science-Strong Future (link). The forum focused on the impact of COVID-19 and how research can help the country emerge stronger.
Breaking Down Barriers for Children with Intellectual and Developmental Disabilities

People living with intellectual and developmental disabilities and their families often face barriers to participating in their own communities, from a building’s design that makes entry difficult to misperceptions when a person’s behavior does not align with social expectations. Through building regional stakeholder communities and a roadmap for future comparative effectiveness research, a project funded by a Eugene Washington PCORI Engagement Award supported efforts to make participation easier for people with intellectual and developmental disabilities and their families.
What is a Financial Conflict of Interest, and why is it important for you as a researcher?
A financial conflict of interest (FCOI) is defined as a significant financial interest (SFI) that could directly and significantly affect the design, conduct, or reporting of sponsored research or non-sponsored research. Identification and responsible management of FCOIs are crucial for safeguarding research objectivity and integrity.

In 2012, ATSU adopted the FCOI in Research policy (No. 20-117) to comply with mandatory Public Health Service regulations for eligibility in receiving grant funding and ability to conduct research. Minor policy updates were approved in July 2020 to boost ATSU’s federal compliance, particularly for non-sponsored human subject research. Changes included:
Policy revisions were guided by a Sponsored Programs FCOI work group in close consultation with ATSU’s Research, Grants, and Scholarly Innovations; Arizona and Missouri Institutional Review Boards; Human Resources; and General Order Review Committee. Additional actions included updates to the University’s IRB forms and the Grant/Contract Application Internal Approval Form.
Looking ahead, FCOI acknowledgements and educational pieces will be incorporated into ATSU’s annual Required Employee Training.
Barbara Edson, BSN, MBA, MHA, executive director of the University of North Carolina Health Care System Virtual Care Center, discussed the journey her institution took in establishing a new telehealth program and several case examples of how teamwork is critical to telehealth workflows for provider eConsults and provider-patient ambulatory video visits.
Sponsored Programs (SP) routinely covers webinars on a wide range of topics tied to ATSU's strategic priorities and is happy to share key takeaways. Summaries of these webinars are available from SP staff upon request (pds@atsu.edu).
| Date | Organization | Webinar |
|---|---|---|
| June 17 | American Hospital Association | “Telehealth: Teams Transform Health Care” |
| July 20 | American Medical Association | "Applying Systems Thinking to Address Structural Racism in Health Professions Education: Curriculum, Structural Competency, and Institutional Change" (recording) |
| August 3 | American Medical Association | “Fostering Agility in Learning: Competency-Based Medical Education and Coaching to Support Master Adaptive Learners” (recording*) |
| August 25 | Health Resources and Services Administration | "The Relative Contribution of Social Determinants on the Health Status of Health Center Patients" |
| September 2 | US Department of Health and Human Services | "Clinical Best Practices & the Art of the Tele-Physical Exam" (recording) |
| September 9 | Weitzman Institute | "Telehealth and Patient Engagement Strategies: The Operation Team Perspective" (recording) |
| September 9 |
American Association of Colleges of Nursing Interprofessional Education Collaborative |
"Practice and Policy in a Pandemic: Accreditation, Regulations, Future Implications" (recording*) |
*These recordings require entering personal information (e.g., email address) for access.
SP plans to cover these webinars/conferences in the next quarter. Please contact us if you would like to be included in the distribution list for any summary.
| Date | Organization | Title |
|---|---|---|
| September 16 | US Department of Health and Human Services | "The New Normal: Tips to Make Telemedicine Part of Your Permanent Practice" (information) |
| September 16-17 | Patient-Centered Outcomes Research Institute |
PCORI Annual Meeting sessions (information):
|
NIH News
The National Advisory Council for Complementary and Integrative Health (NACCIH) will hold its first meeting for FY 2021 on September 25, 2020. Potential grant applicants and other NCCIH stakeholders are invited to attend the livestream from 10:15 am to 3:20 pm EST on NIH Videocast. No registration is necessary.
Key agenda items (full agenda):
Comments (750 words or less) may be emailed to Partap Khalsa, DC, PhD, director of NCCIH Division of Extramural Activities, at khalsap@mail.nih.gov up to 15 days after the meeting.
The Fall 2020 NIH Virtual Seminar on Program Funding and Grants Administration (link) is happening Tuesday, October 27-Friday, October 30. This event is designed to demystify the NIH grant application and review process.
 What to expect:
What to expect:
Register for free today!

The Cancer Moonshot Seminar Series (link) aims to provide a platform for outreach related to Cancer Moonshot initiatives, promote the sharing of knowledge and data from Cancer Moonshot projects, and enhance interactions and collaboration among cancer researchers.
Seminars will generally be held from 12-1 pm EST on the fourth Thursday of each month. View the seminar schedule and register here.
The following NIH funding opportunity announcements (FOAs) will now include explicit criteria defining applications as "non-responsive", or falling outside the scope of the opportunity:
Please note applications deemed non-responsive will be withdrawn from review. Applicants are strongly encouraged to contact the applicable scientific contact listed on the FOA to discuss fit of the proposed project prior to submitting.
 For those planning to submit human subject-related information after peer review (e.g., Just-in-Time, Research Progress Performance Reports) using the eRA Human Subjects System, please keep in mind:
For those planning to submit human subject-related information after peer review (e.g., Just-in-Time, Research Progress Performance Reports) using the eRA Human Subjects System, please keep in mind:
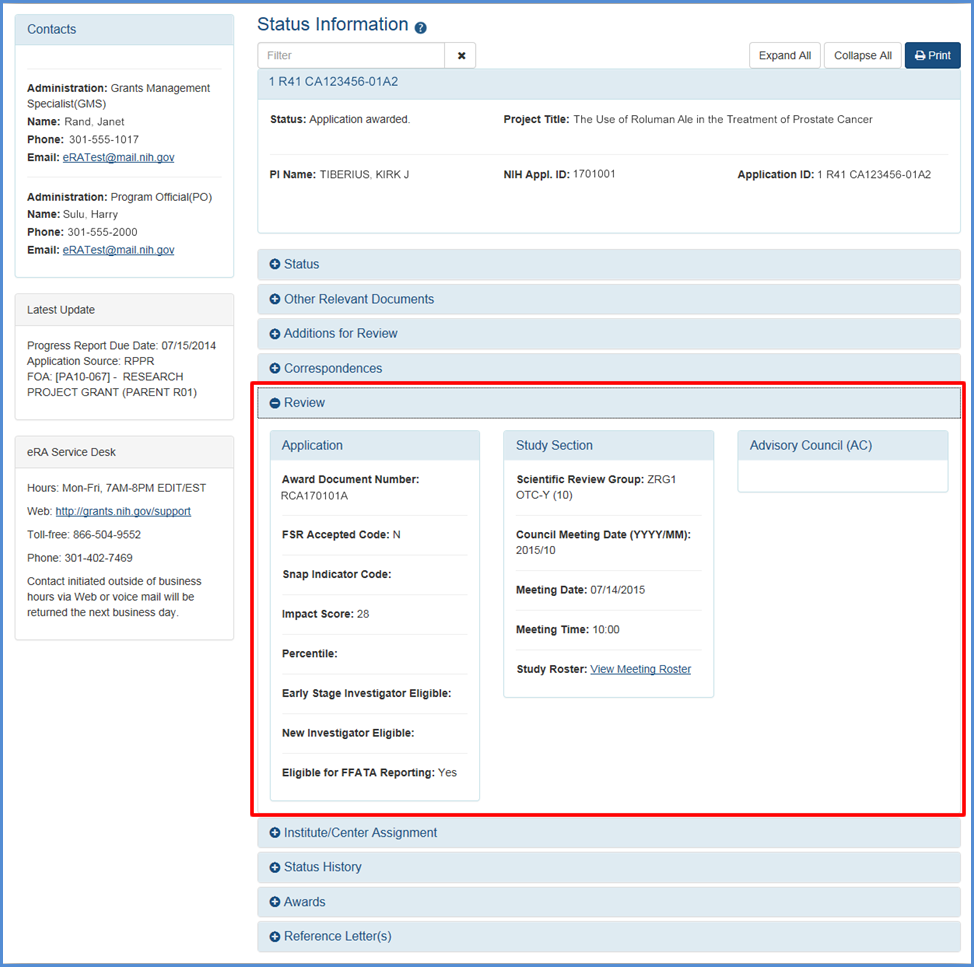
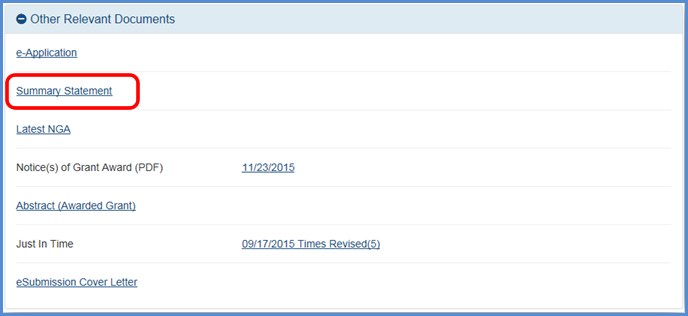
Users with the Signing Official role in the eRA Commons now have access to all current and previously issued overall impact scores and summary statements (link to notice).