☰ ATSU Navigation

In the US, a number of clinical trials were able to proceed despite initial challenges presented by the pandemic by changing longstanding practices. Moving forward, there is growing consensus these changes should be implemented more permanently. Practices adopted in response to the pandemic that would make trials "friendlier" for both patients and staff long term include:
For more information, read this AAMC article.
The National Academies of Sciences, Engineering, and Medicine recently published a new report recommending actionable objectives and strategies for implementing high-quality primary care in the US. Download the free PDF here (must create an account first).
With the NCCIH's new strategic plan, the Center is expanding its definition of integrative health to include whole person health that considers not only the biological but also behavioral, social, and environmental domains of health.
The Center has 5 objectives over the next 5 years:
View the plan here.
In its revised policy, AHRQ expanded its definition of priority populations to include members of the underserved communities specified in Section 2(a) of Executive Order 139895 issued January 20, 2021. The new definition encompasses:
Sponsored Programs routinely covers webinars on a wide range of topics tied to ATSU's strategic priorities and is happy to share key takeaways. Summaries of these webinars are available from Sponsored Programs staff upon request (pds@atsu.edu).
| Date | Organization | Webinar |
|---|---|---|
| March 23 | Patient-Centered Outcomes Research Institute | Virtual Briefing: COVID-19 and the Future of Telehealth |
| March 31 | Missouri Foundation for Health | Messaging to Missourians about the COVID-19 Vaccine |
| March 31 | Primary Care Collaborative | Innovations in Integrating Oral Health into Primary Care |
| April 15 | A.T. Still University College of Graduate Health Studies | Addressing Wellness in Rural Populations: Challenges and Opportunities for the Rural Health Landscape |
| May 13 | Primary Care Collaborative | The National Academies of Sciences, Engineering, and Medicine’s New Report on Primary Care: Recommendations and Reactions |
NIH recently released an updated Strategic Plan for COVID-19 Research (PDF). Learn more at the NIH COVID-19 website.
In its support of COVID-19 research, NIH is guided by 5 key priorities:
Extramural investments in research
In FY 2020, NIH received $41.6 billion in total appropriations from Congress.
Of this amount, $30.8 billion (4.4% increase over FY 2019) went to 56,169 (2.1% increase over FY 2019) new and renewed meritorious extramural grants.
2,650 organizations throughout the US and internationally received awards.
For more statistics on R01-equivalent grants, read this blog post by Mike Lauer, MD, NIH deputy director for extramural research.
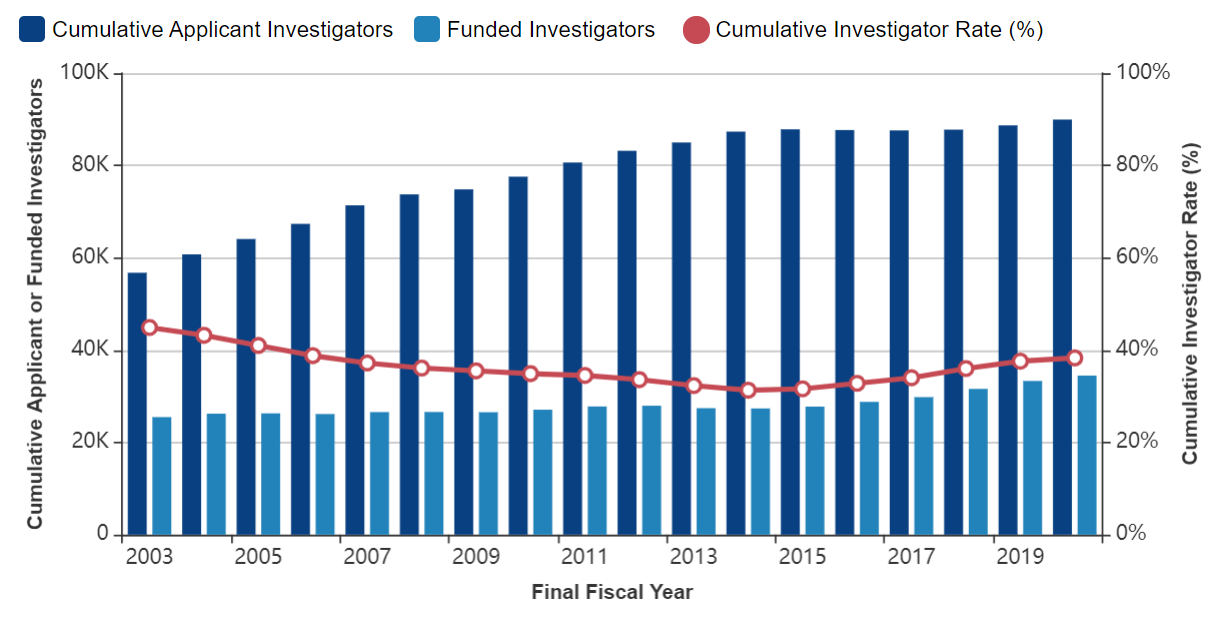
How many researchers were funded?
The “cumulative investigator rate” is a person-based metric that captures the likelihood that unique investigators are funded over a 5-year period.
In FY 2020, the cumulative investigator rate for research project grants was 38.3%.
34,477 awardees (3.5% increase over FY 2019)
89,957 unique applicants (1.4% increase over FY 2019)
Check out this post for statistics on R01-equivalent, P01, and R21 grants.
Please be aware use of the new biosketch and other support format pages and instructions are preferred immediately but required for application due dates on or after January 25, 2022.
Send any questions related to changes to the biosketch and other support templates to the new, dedicated central email inbox at nihosbiosketch@nih.gov.
In annual progress reports, NIH awardees are required to report individual-level study participant data on:
Data must be de-identified and submitted using the required template file.
PDFs that have fillable fields, electronic signatures, text boxes, or images, contain multiple layers. Flattening a PDF merges separate elements into one flat layer. For submissions on or after May 25, 2021, PDFs in the following sections are now required to be flattened for upload to eRA Commons:
See here for instructions on how to flatten a PDF.