The R21 activity code offers a stepping stone for projects that diverge from an investigator’s main thrust of research or support high-risk, high-reward pilot studies. It’s strange to think of an NIH grant activity code as being en vogue, but over the past decade the number of exploratory/developmental grant (R21) applications has continually increased within various NIH Institutes/Centers (ICs), nearly keeping pace with submissions of R01 applications. The following example shows National Institute of Allergy and Infections Disease (NIAID) research project grant applications during the past 10 years.
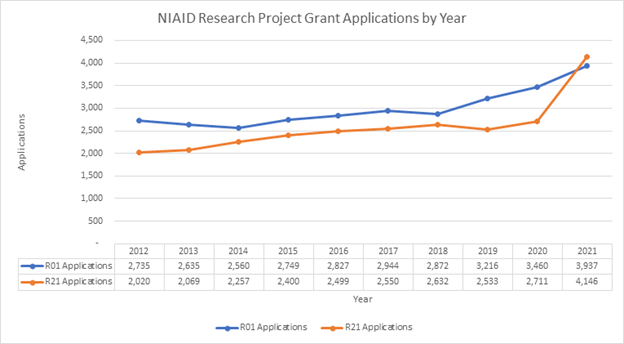
Includes applications for emergency coronavirus-related initiatives. Credit: NIAID
Administrative Basics: The R21 activity code provides up to 2 years of funding support. Typically, direct costs for the entire project period are capped at $275,000, with no more than $200,000 requested for any single year of the project period. Applicants must use the PHS 398 Modular Budget Form. NIH’s Standard Due Dates for investigator-initiated R21 applications are February 16, June 16, and October 16 (AIDS and AIDS-related R21 application due dates differ). Not all NIH institutes and centers participate in the R21 parent FOAs, which could limit the awarding components available for you to request on the PHS Assignment Request Form but would not constrain your study section assignment preferences.There are two parent funding opportunity announcements (FOAs) for R21 grants: NIH Exploratory/Developmental Research Grant Program (Parent R21, Clinical Trial Not Allowed) and NIH Exploratory/Developmental Research Grant Program (Parent R21, Clinical Trial Required). Parent R21 FOAs do not allow renewal applications.
Programmatic Niche: The R21 activity code’s administrative aspects complement its programmatic purpose: To support innovative exploratory/developmental research by providing support for the early and conceptual stages of project development. The R21’s short duration and limited scope is ideal for investigators to introduce novel scientific ideas, model systems, tools, agents, targets, and technologies that have the potential to substantially advance biomedical research. Unlike the R01 parent FOAs, which require you to provide preliminary data in your application, the R21 FOAs allow but do not require preliminary data. This difference underscores the R21’s prioritization of new scientific approaches.
A small project of similar scope to an R21 but using widely accepted approaches and methods is better suited for the small grant (R03) activity code. Examples of projects too narrow for an R21 application but ideal for an R03 grant include secondary analysis of existing data, descriptive studies, and surveys.
Strategic Considerations: Of course, the goal of an applicant is to score well in peer review and receive funding. Whether because its budget is smaller, it doesn’t require preliminary data, or it doesn’t negate early-stage investigator status, there is a tendency among newly independent researchers to assume the R21 grant is easier to get than an R01 grant and thus an ideal career building block. But those suppositions are false. The R21 is not designed to be a stepping stone for research career development—it’s meant to be a stepping stone for projects that diverge from an investigator's main thrust of research or support high-risk, high-reward pilot studies. Many NIH ICs offer a preferential payline for new and early-stage investigators applying for an R01 grant, which is not the case for an R21 grant. The R01 offers five years of funding and can be renewed following a productive project period, which sets a foundation that the R21 cannot match.
While trends vary by IC, funding success rates for R21 grants are typically not meaningfully better than success rates for R01 grants. Shoehorning a project into an R21 application when its Research Strategy and Specific Aims truly necessitate a longer project period or a larger budget will not improve your odds of funding. You should always apply using the activity code that, if funded, gives your research project its highest likelihood of success.



